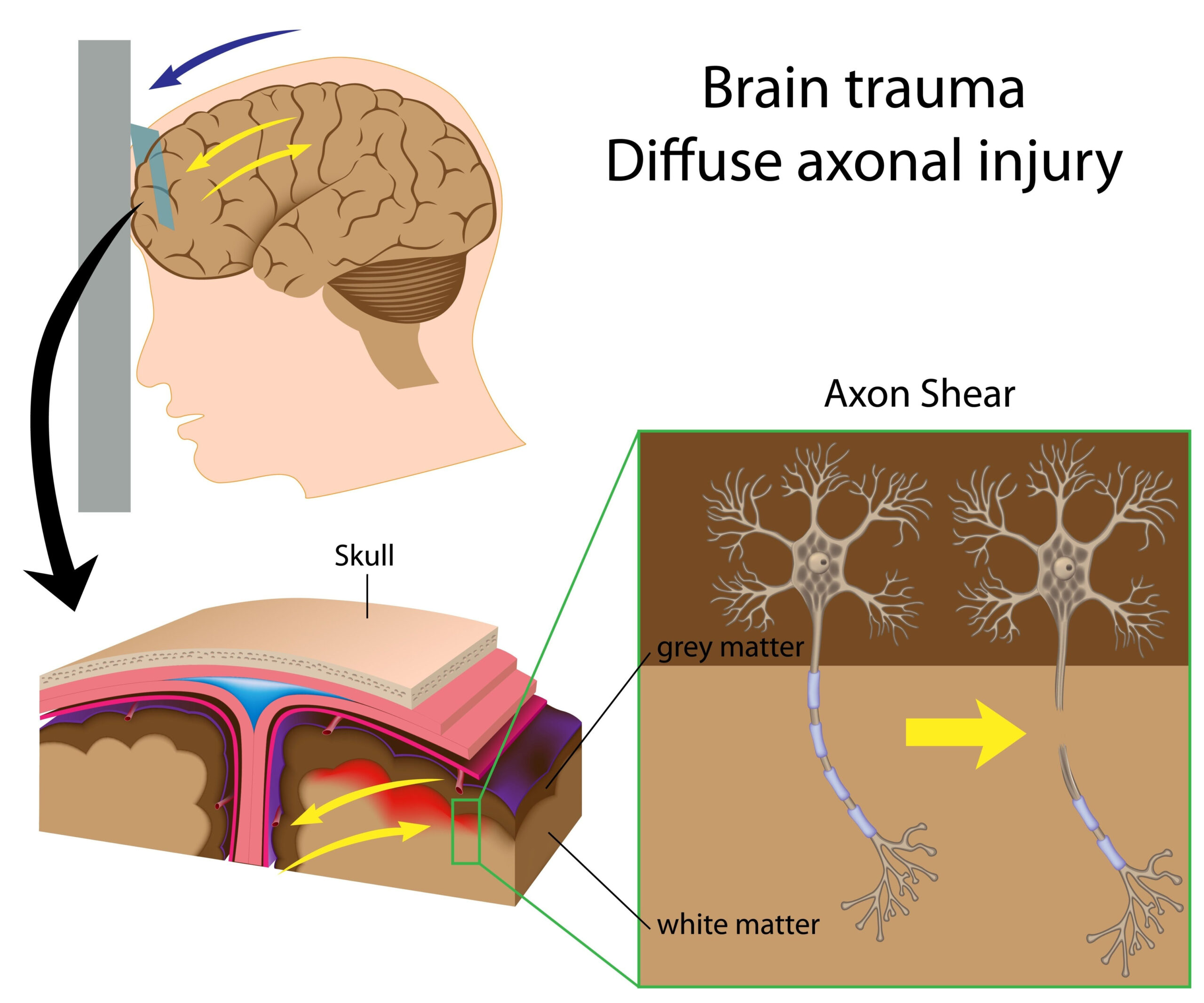
Biomarkers in cerebrospinal fluid analysis
Cerebrospinal fluid (CSF) serves as a crucial medium for assessing the biochemical and physiological state of the central nervous system (CNS). Following a traumatic brain injury (TBI), the analysis of metabolites and biomarkers in CSF can provide significant insights into the extent of injury and potential secondary complications. Biomarkers are typically defined as biological molecules found in tissues, cells, or bodily fluids that indicate a normal or abnormal process or condition. In the context of TBI, specific metabolites in CSF can reflect neuronal damage, inflammation, and glial cell activation, contributing to our understanding of injury mechanisms and recovery processes.
Several key types of biomarkers can be identified in CSF following TBI. Neurotransmitters, for instance, can provide information about neuronal function following injury. Elevated levels of certain neurotransmitters or their metabolites may indicate excessive neuronal activity or excitotoxicity, a process where neurons are damaged by high levels of excitatory signals. Moreover, proteins associated with inflammation, such as cytokines and chemokines, often see increased concentrations in CSF after TBI. These changes signal an inflammatory response aimed at repairing the injured brain, yet if dysregulated, this inflammation can also lead to further injury and neurodegeneration.
Another important class of biomarkers consists of specific metabolites associated with metabolic pathways altered by TBI. For example, changes in lactate concentration can indicate disruptions in energy metabolism, suggesting a shift towards anaerobic metabolism due to decreased oxygen supply to brain tissues. Other metabolites, such as creatine, N-acetylaspartate (NAA), and choline, are also monitored as they reflect various aspects of neuronal integrity and metabolism. NAA, in particular, is recognized as a marker for neuronal health; diminished levels may indicate neuronal loss or dysfunction.
In addition to individual markers, comprehensive metabolomic profiling of CSF can enhance the discovery of biomarker panels that collectively provide a more nuanced picture of TBI severity and the potential risk for secondary injuries. Advanced analytical techniques, such as mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy, have revolutionized metabolomic analysis, enabling researchers to detect and quantify hundreds of metabolites simultaneously. This high-throughput approach opens new avenues for identifying reliable and predictive biomarkers for clinical assessment and monitoring post-injury recovery.
Ultimately, the evaluation of biomarkers in CSF following TBI contributes significantly to our understanding of injury dynamics and holds promise for developing targeted therapies and intervention strategies. The identification and validation of these biomarkers are essential for advancing personalized medicine and improving prognostic capabilities in traumatic brain injuries, paving the way for better management of affected individuals.
Study design and sample collection
The investigation into the metabolomic alterations in cerebrospinal fluid (CSF) following traumatic brain injury (TBI) necessitates a carefully structured study design to ensure the reliability and relevance of the findings. A well-defined strategy includes selecting an appropriate cohort of participants, establishing clear inclusion and exclusion criteria, and optimizing sample collection and handling procedures.
Participants in this study typically include patients diagnosed with TBI, specifically those presenting at emergency departments within a specified timeframe following injury. This approach allows researchers to capture the acute phase of injury, which is essential for understanding the immediate biochemical responses occurring within the CNS. Inclusion criteria might include various severity levels of TBI, determined by clinical assessments or imaging studies, while exclusion criteria could encompass pre-existing neurological disorders, concurrent systemic illnesses, or conditions that influence CSF composition, such as infections or metabolic abnormalities.
Sample collection is a critical component of this research design. CSF samples are usually obtained through lumbar puncture, a minimally invasive procedure that allows for the direct extraction of fluid from the subarachnoid space. Timing of sample collection relative to the injury is vital; samples should ideally be collected shortly after injury and at later time points to capture dynamic changes in metabolomic profiles. This longitudinal approach enables an assessment of how biomarkers evolve over time and correlate with clinical outcomes.
To maintain the integrity of the samples, strict protocols must be followed during the collection and processing stages. This includes the use of sterile techniques to prevent contamination, as well as immediate processing of CSF to separate the cellular components from the fluid. Careful aliquoting of the CSF into multiple storage containers and rapid freezing at -80°C are standard practices that help preserve the metabolites of interest for subsequent analysis.
Upon collection, advanced metabolomic techniques, such as liquid chromatography coupled with mass spectrometry (LC-MS) or gas chromatography-mass spectrometry (GC-MS), are employed to profile the CSF metabolites. These methodologies allow researchers to detect a wide array of small molecules, including amino acids, lipids, and energy metabolites, that may serve as biomarkers for neuronal injury or dysfunction.
In sum, the combination of a meticulously planned study design with rigorous sample collection protocols provides a robust framework for investigating the metabolomic landscape of CSF following TBI. This methodological rigor is essential for identifying potential biomarkers that could inform clinical practice and enhance our understanding of TBI’s pathophysiology.
Results and interpretation of metabolomic profiles
The analysis of cerebrospinal fluid (CSF) utilizing advanced metabolomic techniques has revealed substantial shifts in metabolite concentrations following traumatic brain injury (TBI). These changes not only serve as indicators of neuronal injury but also provide insights into the broader pathological processes that occur after trauma. The results from metabolomic profiling have identified various significant metabolites that correlate strongly with TBI severity, recovery outcome, and the development of secondary injuries.
In cases of mild to moderate TBI, alterations in metabolite levels often reflect subtle disruptions in neuronal integrity and function. For instance, N-acetylaspartate (NAA) concentrations typically decrease in relation to increased neuronal loss or dysfunction, marking it as a crucial marker of neurodegeneration. Notably, lower NAA levels in CSF have been correlated with worse clinical outcomes, illustrating its potential utility in prognostic assessments following injury.
In addition, researchers observed significant elevations in lactate levels, which indicate a shift towards anaerobic metabolism in response to reduced oxygen supply to brain tissue. This metabolic acidosis can be detrimental, contributing to excitotoxicity and neuronal damage if prolonged. Elevated lactate levels in acute TBI instances may thus signal metabolic distress and highlight the critical window for therapeutic intervention.
Furthermore, increases in various inflammatory markers, such as cytokines and chemokines, were detected in CSF samples from TBI patients, supporting the role of the immune response in the pathophysiology of brain injury. Elevated concentrations of specific pro-inflammatory cytokines have consistently been shown to correlate with the severity of clinical symptoms and can suggest ongoing neuroinflammation, which may exacerbate injury and complicate recovery.
The comprehensive metabolomic profiles have enabled the identification of distinct biomarker panels consisting of both metabolic and inflammatory indicators. Analyses have demonstrated that specific combinations of metabolites can yield improved sensitivity and specificity when predicting outcomes compared to single biomarkers. For instance, a panel that combines NAA, lactate, and selected inflammatory mediators provides a multifaceted view of the underlying processes and potential complications arising from TBI.
A critical aspect of the interpretation of these metabolomic profiles lies in the temporal dynamics following injury. Longitudinal studies have shown that certain biomarkers fluctuate at different time points post-injury, reflecting the dynamic nature of the body’s response. Early elevation of inflammatory markers might suggest a protective response, while sustained high levels may indicate a maladaptive response leading to further cellular injury.
In summary, the results of metabolomic profiling in CSF after TBI underscore the intricate relationship between metabolic shifts and the inflammatory response. These findings not only enhance our understanding of TBI pathophysiology but also open avenues for the development of targeted therapeutic strategies. Identifying and validating these metabolomic biomarkers could potentially lead to improved monitoring of injury and more personalized approaches to treatment, ultimately aiming to mitigate the impact of secondary injuries and enhance recovery trajectories in patients with TBI.
Future directions in traumatic brain injury research
The future of traumatic brain injury (TBI) research is bright, with an increasing focus on the integration of metabolomic analyses into clinical practice. As we build upon existing knowledge, several promising avenues warrant further exploration. One of the most critical aspects is the refinement and validation of biomarker panels identified through cerebrospinal fluid (CSF) analysis. Establishing standardized protocols for biomarker assessment is essential to ensure consistency across diverse clinical settings. This standardization could significantly enhance the reliability of prognostic evaluations and treatment plans tailored to individual patients.
Moreover, longitudinal studies that track metabolomic changes over time will be crucial for elucidating the timeline of injury progression and recovery. Understanding how specific metabolites fluctuate during the acute, sub-acute, and chronic stages of TBI can provide insights into the physiological responses that predetermine recovery trajectories. This knowledge will facilitate the development of intervention strategies aimed at mitigating secondary injuries and improving long-term outcomes.
Advancements in technological methodologies represent another exciting frontier in TBI research. Emerging tools such as single-cell metabolomics and advanced imaging techniques could provide granular insights into the cellular and molecular dynamics within the CNS post-injury. Investigating the interactions between various cell types, including neurons, astrocytes, and microglia, will deepen our comprehension of the pathological mechanisms driving TBI. Furthermore, the application of machine learning algorithms to metabolomic data holds great potential for uncovering hidden patterns and predicting patient outcomes with greater accuracy.
Collaboration across multidisciplinary fields, including neurology, biochemistry, and bioinformatics, is equally essential. Establishing partnerships within the scientific community will foster an environment of shared knowledge and resource pooling, accelerating the pace of discovery. Engaging with patients and their families throughout the research process can also yield invaluable perspectives, ensuring that studies remain relevant and focused on the needs of those affected by TBI.
As we look ahead, the potential for personalized medicine in TBI is particularly compelling. By leveraging detailed metabolomic profiles, clinicians could tailor therapeutic approaches that are specifically designed to target an individual’s unique metabolic and inflammatory responses to injury. This shift towards personalized care represents a paradigm change, providing hope for more effective management and rehabilitation outcomes.
Incorporating biomarkers from CSF into clinical trials will be fundamental in validating their predictive value. Evidence obtained from clinical trials can not only confirm the utility of these biomarkers in real-world settings but also facilitate the approval of novel therapeutic agents aimed at modifying disease progression following TBI. Through rigorous testing and validation, researchers can progressively enhance the toolkit available to clinicians, ultimately leading to improved patient care.
In summary, as we move forward in TBI research, the promise of metabolomics to unearth critical biomarkers reflects a broader shift toward more effective and personalized approaches to treatment. Continued investment in research and collaboration, combined with advancements in analytical technologies, lays the foundation for transformative breakthroughs in understanding and managing traumatic brain injuries.