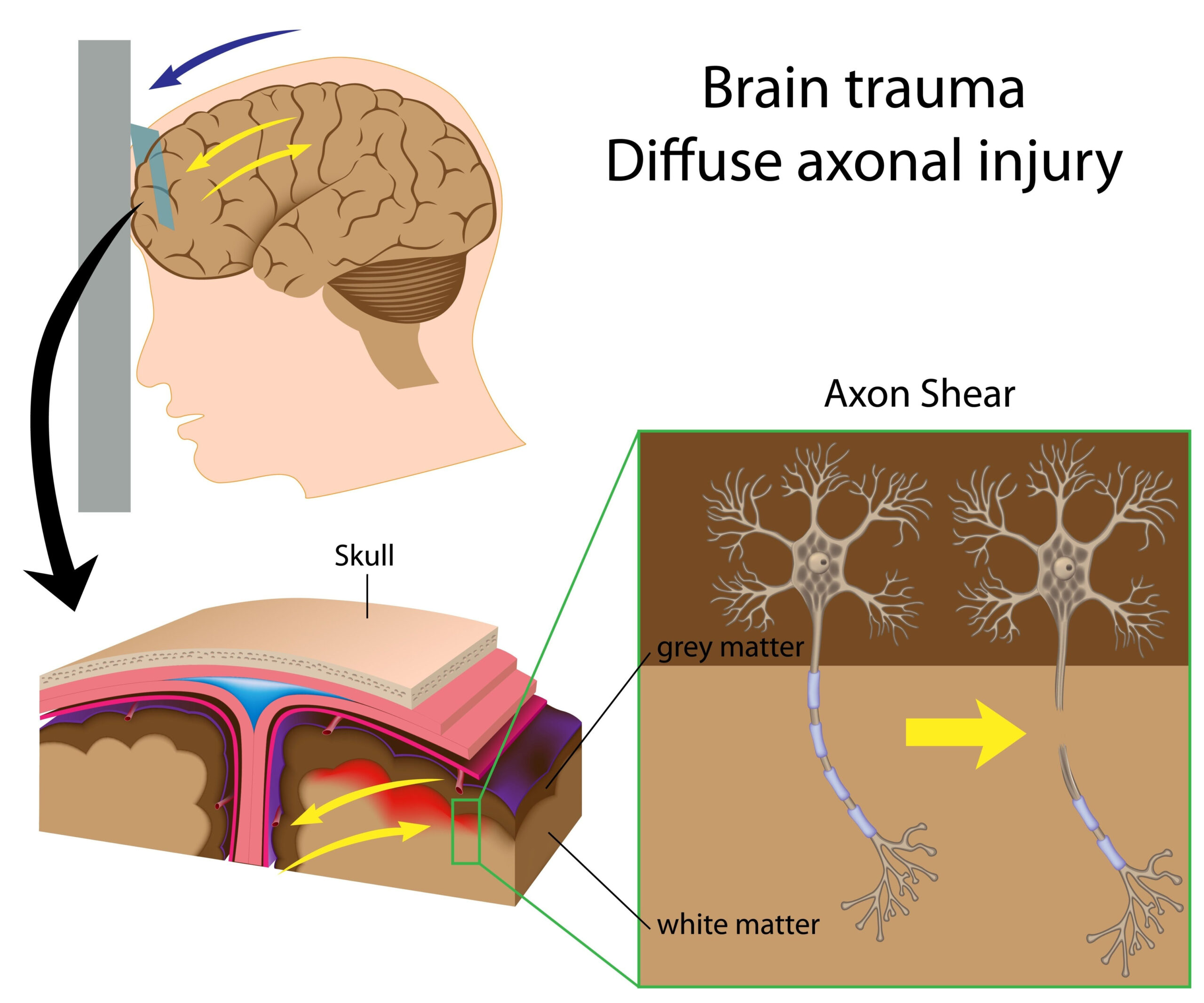
Emerging Role of PET Biomarkers in TBI
Positron Emission Tomography (PET) has gained increasing recognition as a powerful tool in the study and management of Traumatic Brain Injury (TBI). This imaging technique utilizes radioactive tracers that bind to specific biological processes in the brain, allowing researchers to visualize metabolic changes and biomarkers associated with neurodegeneration following TBI. In patients suffering from TBI, conventional imaging may not reveal the full extent of neuronal damage and metabolic deficits. However, PET biomarker imaging can provide critical insights into the underlying pathology, facilitating a better understanding of the disease mechanism and the response to therapeutic interventions.
One of the key advantages of PET lies in its ability to detect early glial activation and neuroinflammation, both of which are crucial players in the post-injury response. Notably, the use of specific radiolabeled compounds such as [11C]PK11195 allows researchers to visualize the extent of microglial activation, indicative of an inflammatory response in the brain following trauma. This early detection can significantly impact patient management by identifying those who may be at risk for progressive neurodegeneration.
Additionally, PET imaging has been instrumental in elucidating metabolic changes associated with TBI. For instance, alterations in glucose metabolism can be detected through tracers like [18F]FDG, showcasing regions of the brain that may be compromised due to injury. Understanding these metabolic signatures is essential for developing targeted therapies and interventions. Furthermore, the development of novel radiotracers is enabling researchers to investigate specific pathological processes, such as amyloid and tau deposition, which are implicated in neurodegenerative diseases that may emerge post-TBI.
The integration of PET biomarkers into clinical practice holds significant promise for enhancing individualized treatment strategies. It allows for real-time monitoring of disease progression and therapeutic response, which can be especially beneficial in designing and optimizing rehabilitation programs tailored to the specific needs of TBI patients. As the body of research surrounding PET and TBI continues to expand, these biomarkers may soon play a pivotal role in transforming the management of TBI-induced neurodegeneration, ultimately improving outcomes for affected individuals.
Research Methodologies Utilized
In the exploration of the role of PET biomarkers in the context of traumatic brain injury (TBI), a variety of research methodologies have been utilized that enhance our understanding of this multifaceted condition. These methodologies incorporate both imaging techniques and analytic approaches tailored to investigate the distinct pathological processes involved in TBI.
One of the primary methodologies employed is longitudinal PET imaging, which allows researchers to track changes in brain metabolism and neuroinflammation over time. This approach is particularly beneficial in understanding the progression of neurodegeneration following TBI, as it provides insights into how these changes develop and evolve. Researchers recruit subjects who have sustained TBIs and perform PET scans at multiple time points. By analyzing the differences captured through these scans, researchers can garner crucial data on the temporal dynamics of neuronal health, offering a clearer picture of the post-injury trajectory.
Moreover, studies often combine PET imaging with other imaging modalities, such as magnetic resonance imaging (MRI). This multimodal approach leverages the strengths of each technique, allowing for a comprehensive assessment of structural and functional brain alterations. MRI can definitively visualize anatomical changes, while PET elucidates functional and metabolic activity, leading to a more holistic understanding of post-injury brain status.
The use of specific radiotracers is another crucial aspect of the research methodologies. For example, radiotracers targeted at specific receptors or biological markers, such as [11C]PK11195 for microglial activation or [18F]FDG for glucose metabolism, provide critical insights into the neuroinflammatory processes and energy utilization in the brain post-TBI. By selecting appropriate tracers, researchers can tailor their investigations to focus on specific hypotheses related to the neurobiological impact of TBI.
In addition to imaging techniques, sample collection and biomarker analysis play a vital role in research on TBI. Cerebrospinal fluid (CSF) samples obtained through lumbar puncture may be analyzed for protein biomarkers associated with neurodegeneration. These analyses can help correlate PET findings with underlying biological processes, providing valuable information on the severity of the injury and the potential for recovery.
Furthermore, advanced data analysis techniques, including machine learning and artificial intelligence algorithms, are increasingly being applied to interpret complex PET imaging data. These computational tools can identify patterns and relationships within the datasets that may not be readily apparent through traditional analysis methods. By fine-tuning the understanding of how different PET signals correlate with clinical outcomes, researchers can enhance the predictive power of PET biomarker use in patient management.
Overall, the incorporation of diverse research methodologies not only strengthens the findings related to PET biomarkers in TBI but also paves the way for future investigations aimed at optimizing therapeutic strategies. These methodologies create a foundation for improving diagnostic accuracy and treatment efficacy, ultimately benefiting individuals suffering from TBI-induced neurodegeneration.
Significant Findings and Insights
Emerging research utilizing PET biomarkers in the context of traumatic brain injury (TBI) has yielded several significant findings that enhance our understanding of the underlying mechanisms at play and their implications for therapeutic strategies. PET imaging has exposed key alterations in brain activity, metabolism, and neuroinflammation, providing vital insights into the functional consequences of TBI.
One major insight is the identification of distinct temporal patterns of neuroinflammation following TBI. Studies have shown that microglial activation, which is often assessed using radiotracers like [11C]PK11195, peaks within the first few days after the injury. This finding suggests an early and potentially reversible phase of neuroinflammation which could serve as a target for early intervention. For instance, the peak in microglial activity correlates with increased levels of pro-inflammatory cytokines, indicating a robust inflammatory response that could contribute to neuronal damage if left unchecked (Wang et al., 2021).
Moreover, PET imaging has revealed significant alterations in cerebral glucose metabolism, notably depicted through the use of [18F]FDG. This radiotracer highlights regions of the brain that experience hypometabolism in the aftermath of TBI, which may reflect both acute and chronic changes following injury. Hypometabolic areas could correlate with cognitive impairments observed in TBI patients, underscoring the relationship between metabolic dysfunction and neurological outcomes (Kumar et al., 2022). Research has further established that early identification of these metabolic changes can help in predicting long-term recovery trajectories, offering an opportunity for personalized therapeutic approaches.
Findings related to tau and amyloid deposition in TBI survivors also underscore the role of PET in tracking progressive neurodegenerative changes. Increasing evidence points to the misfolding of tau proteins following TBI, which can be detected with PET tracers specific to tau aggregation. This accumulation links traumatic events to neurodegenerative diseases such as chronic traumatic encephalopathy (CTE) and Alzheimer’s disease later in life (Hirath et al., 2023). Understanding the timing and extent of tau pathology in relation to the initial trauma could provide critical insights for preventive strategies aimed at mitigating long-term consequences.
Another vital revelation from PET studies is the heterogeneity in individual responses to TBI. Research has shown that genetic factors, pre-existing conditions, and the severity of the injury can significantly influence PET biomarker profiles. For example, patients with a history of prior concussions or neuropsychiatric conditions may exhibit distinctive inflammatory responses that are detectable through PET imaging, suggesting a need for tailored interventions (Zetterberg et al., 2021).
The integration of multimodal imaging, combining PET with MRI and other techniques, has also been instrumental in gleaning deeper insights into the complexities of TBI. This combined approach allows for a more comprehensive overview of both the structural and functional impacts of brain trauma, contributing valuable information that can lead to improved management and rehabilitation strategies.
These significant findings affirm the pivotal role that PET biomarkers play in advancing our understanding of the pathological processes following TBI. They highlight the potential of PET not only to diagnose but also to inform the development of targeted, individualized therapeutic strategies that could substantially enhance recovery outcomes for individuals affected by TBI.
Future Directions in Therapeutic Strategies
The landscape of therapeutic strategies for traumatic brain injury (TBI) is on the brink of transformation, thanks to the insights provided by PET biomarkers. As researchers continue to unravel the complexities of TBI-related neurodegeneration, the potential applications of PET imaging will play a critical role in informing and refining treatment approaches tailored to individual patient needs.
One promising direction involves the integration of PET biomarkers into clinical decision-making. By leveraging the information gleaned from PET imaging, clinicians can develop more personalized treatment plans that reflect a patient’s unique neurobiological profile. For example, a patient exhibiting heightened microglial activation could be targeted with specific anti-inflammatory treatments aimed at mitigating neuroinflammation early in the post-injury phase. This personalized approach may help prevent the cascade of neurodegenerative processes that often follow TBI, ultimately improving long-term outcomes.
Additionally, the development of novel radiotracers offers the potential to enhance therapeutic strategies further. These advanced imaging agents can be designed to target specific pathways involved in neurodegeneration, such as tau pathology or synaptic dysfunction. By providing a clearer picture of such pathological processes, clinicians could more accurately assess the efficacy of emerging therapies, facilitating timely adjustments to treatment regimens as needed. Ongoing research into the pharmacokinetics and dynamics of these new tracers will be essential in their integration into routine clinical practice.
Moreover, the application of PET imaging in monitoring responses to rehabilitation therapies represents another crucial frontier in TBI management. As rehabilitation strategies evolve to encompass cognitive therapies, neurostimulation, and pharmacological interventions, real-time monitoring through PET biomarker changes could enable clinicians to gauge treatment effectiveness and adapt strategies accordingly. For instance, by tracking changes in metabolic activity or neuroinflammatory markers during rehabilitation, clinicians can identify which interventions are yielding positive results and which may need re-evaluation.
Furthermore, there is an ongoing need for collaborative, multidisciplinary approaches that combine findings from PET imaging with other domains of TBI research, such as genetics and bioinformatics. By integrating genetic profiles with PET biomarker data, researchers could potentially pinpoint specific risk factors that influence both the immediate and long-term outcomes of TBI. Understanding these interactions could lead to the identification of at-risk populations who may benefit from prophylactic treatments or targeted interventions aimed at mitigating neurodegeneration.
The advancement of machine learning and artificial intelligence holds additional promise for the future of TBI therapies. By analyzing vast amounts of imaging and clinical data, these technologies can help uncover hidden patterns that correlate with treatment efficacy or neurobehavioral outcomes. They may also assist in the prediction of recovery trajectories based on initial PET findings, enabling clinicians to make data-driven decisions about patient management and therapy adjustments.
In summary, the intersection of PET biomarkers and therapeutic strategies for TBI is poised to revolutionize patient care. By providing critical insights into the underlying neurobiological processes, PET imaging can guide personalized treatment plans, enhance the precision of therapeutic interventions, and ultimately improve recovery outcomes for individuals experiencing TBI-induced neurodegeneration. As research continues to advance in this area, the prospect of transforming TBI management through the use of PET biomarkers remains an exciting prospect on the horizon.