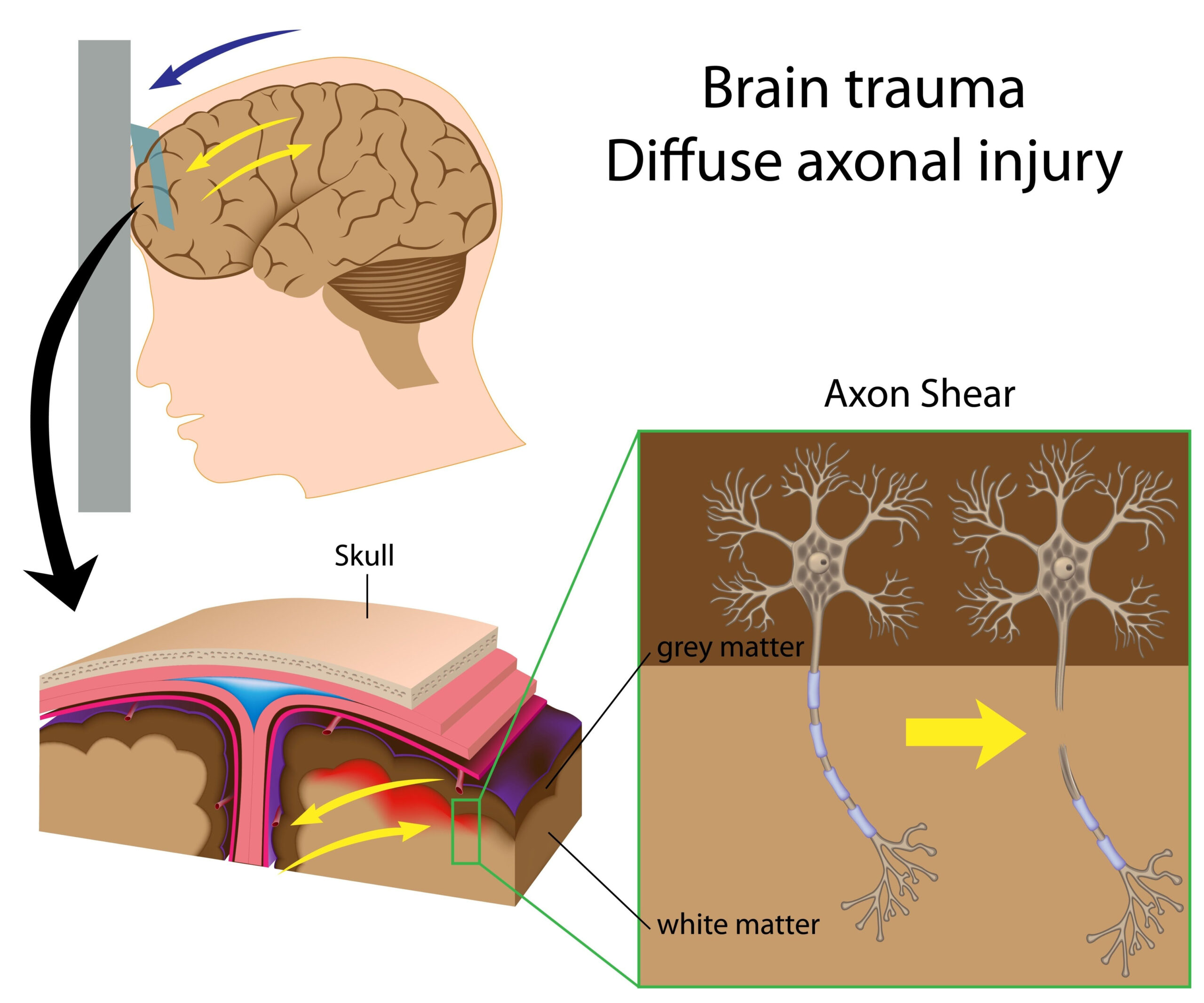
Metabolomic Profiling
Metabolomic profiling involves the comprehensive analysis of metabolites—small molecules that are the end products of cellular processes—in biological samples. In the context of traumatic brain injury (TBI), this approach is pivotal for identifying biochemical changes that occur in cerebrospinal fluid (CSF) following an injury. CSF serves as a unique medium, providing insights into the central nervous system’s (CNS) metabolic responses to trauma.
Through advanced analytical techniques such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS), researchers can obtain detailed profiles of metabolites in CSF samples. These techniques enable the detection of a wide array of metabolites, including amino acids, lipids, nucleotides, and various signaling molecules. Profiling the metabolome can reveal alterations linked to biochemical pathways that may contribute to secondary injuries post-TBI. For instance, changes in amino acid concentrations can indicate shifts in neurotransmitter production or energy metabolism alterations, both critical in understanding the brain’s response to injury.
The metabolomic alterations observed in TBI can provide valuable insights into the timing, severity, and possible prognosis of the injury. Studies have shown that specific metabolites correlate with neurological function, allowing for the potential identification of biomarkers that signify injury severity. Identifying such biomarkers could enhance diagnostic accuracy and guide therapeutic interventions tailored to individual patient needs.
Additionally, the integration of metabolomic data with other omics technologies, such as genomics and proteomics, could yield a more holistic understanding of the complex biological responses involved in TBI. This integrated approach provides a better characterization of the injury landscape, paving the way for personalized medicine strategies in treating patients with TBI. Overall, metabolomic profiling stands at the forefront of research aimed at discerning the complex biochemical changes following traumatic brain injuries, enabling better assessment and management of these critical conditions.
Sample Collection and Analysis
The successful application of metabolomic profiling in understanding traumatic brain injury (TBI) hinges significantly on the meticulous procedures involved in sample collection and analysis. Cerebrospinal fluid (CSF) is primarily obtained via lumbar puncture—a procedure requiring aseptic technique to minimize contamination risks. This process allows for direct access to the interstitial fluid of the central nervous system, making CSF an ideal candidate for capturing real-time biochemical changes post-injury.
Once collected, it is imperative that CSF samples are processed quickly and stored appropriately to preserve metabolite integrity. Generally, samples should be centrifuged immediately to remove cellular debris, then stored at low temperatures, typically at -80°C, until analysis. This ensures that the metabolites remain stable, thereby preventing degradation that could otherwise skew results.
Following sample preparation, advanced analytical techniques are employed to characterize the metabolomic profile distinctly. Mass spectrometry (MS) is one of the most frequently utilized methods due to its sensitivity and ability to analyze complex mixtures simultaneously. In TBI research, MS can identify and quantify metabolites based on their mass-to-charge ratios. When coupled with chromatography techniques, such as gas chromatography (GC) or liquid chromatography (LC), researchers can separate these metabolites, improving the resolution and accuracy of identification further.
Additionally, nuclear magnetic resonance (NMR) spectroscopy serves as another powerful analytical technique that can provide information about the molecular structure and concentration of metabolites. While NMR typically has lower sensitivity compared to MS, its advantage lies in non-destructive analysis and the ability to study metabolites in their natural state within the CSF matrix. This characteristic is crucial for identifying a comprehensive spectrum of metabolites, particularly those that may exist in low concentrations.
Analyzing metabolomic data involves sophisticated bioinformatics tools that can handle the complexities of the generated data sets. Various software programs enable researchers to extract meaningful patterns and associations within metabolite profiles, assisting in the identification of potential biomarkers linked to injury severity and outcomes. Statistical analyses often accompany these computational methods to establish correlations between metabolic changes and clinical parameters, aiding in understanding the pathophysiological processes following TBI.
Notably, inconsistencies in sample collection and analysis protocols can introduce variability into metabolomic studies. Therefore, standardization of procedures is essential to ensure reproducibility and comparability of results across different research labs and clinical settings. Collaborative efforts aimed at creating unified guidelines for CSF sample handling, storage, and analysis will enhance the reliability of metabolomic data, facilitating broader application in clinical practice and research alike.
These efforts not only strengthen the foundation of metabolomic research but also extend the potential to translate findings into clinical applications. By integrating metabolomic data with clinical information, healthcare professionals can better understand individual patient profiles, leading to more tailored therapeutic strategies aimed at mitigating secondary injuries and improving patient outcomes after traumatic brain injury.
Biomarkers of Severity
Identifying biomarkers that correlate with the severity of traumatic brain injury (TBI) is a critical area of research within the field of metabolomics. These biomarkers can provide pivotal insights into the extent of the injury, guiding therapeutic strategies and improving patient management. The metabolites found in cerebrospinal fluid (CSF) have emerged as promising indicators of injury severity, with specific changes in their concentrations offering clues about the underlying pathological processes.
For instance, alterations in the levels of amino acids, such as glutamate and aspartate, may signal excitotoxicity—a condition leading to cell damage or death due to excessive neurotransmitter activity. This mechanism is particularly relevant in TBI, where initial mechanical impact and subsequent secondary injury processes can result in heightened excitatory neurotransmission. Elevated levels of these metabolites in CSF could, therefore, serve as biomarkers that reflect the severity of neuronal damage and the risk of further neurological decline.
Furthermore, lipid metabolism changes have been associated with TBI severity. Markers such as phosphatidylinositols, which play critical roles in cell signaling and membrane integrity, can reveal insights into the extent of cellular disruption following injury. Increased concentrations of certain inflammatory lipids, such as lysophosphatidic acid, have been linked to neuroinflammation—a common consequence of TBI that can exacerbate damage and contribute to long-term complications. Tracking these lipid metabolites could help distinguish between mild and severe forms of TBI, enabling better resource allocation and intervention planning.
In addition to amino acids and lipids, metabolites involved in energy metabolism, like lactate and pyruvate, pose significant relevance. An elevated lactate-to-pyruvate ratio in CSF might reflect how the brain’s metabolic state shifts from aerobic to anaerobic processes, indicating a more severe metabolic crisis following TBI. Such an energy imbalance can intensify injury outcomes, thus providing a crucial marker for clinicians to assess functional prognosis and therapeutic needs.
The integration of metabolomics data with clinical outcomes and imaging findings has further amplified the potential of these biomarkers. For example, correlating metabolite levels with neuroimaging results from techniques such as MRI can offer a more nuanced understanding of injury dynamics. This approach helps validate metabolomics findings and assists in constructing predictive models for patient recovery trajectories.
Moreover, emerging technologies in artificial intelligence and machine learning are being utilized to enhance biomarker discovery from metabolomic data. By employing multifactorial analyses of metabolomic profiles alongside demographic, clinical, and imaging data, researchers can facilitate the development of risk stratification tools to aid healthcare professionals in clinical decision-making.
It is noteworthy that while substantial strides have been made in identifying potential biomarkers of severity, further validation in larger, diverse cohorts is critical to establish their clinical utility reliably. Future studies must also focus on standardizing metabolomic measurement protocols and exploring the biological significance of identified metabolites. As researchers continue to unravel the complexities of metabolic changes post-TBI, the goal remains to translate these findings into clinical applications that can inform treatment pathways and ultimately improve outcomes for patients suffering from traumatic brain injuries.
Future Directions in Research
Research in the metabolomics of cerebrospinal fluid (CSF) following traumatic brain injury (TBI) holds significant potential for advancing our understanding of the complex biochemical alterations that occur following an injury. One critical direction for future studies is the exploration of longitudinal metabolomic profiling, where repeated CSF sampling could help elucidate the temporal dynamics of metabolic alterations. This approach could provide insights into how metabolite concentrations change over time, which is vital for understanding acute versus chronic phases of TBI and could enhance the identification of biomarkers for prognosis and recovery.
Enhancing analytical methodologies represents another exciting avenue for future research. The development of more sensitive and specific detection technologies can improve the discovery of low-abundance metabolites that may have significant implications for TBI. Furthermore, advancements in data acquisition techniques and bioinformatics tools will enable researchers to analyze complex metabolomic data sets more effectively, facilitating the identification of subtle but clinically relevant changes in metabolite profiles.
Interdisciplinary collaborations are also vital, incorporating insights from neurobiology, computational biology, and clinical practices. A multidisciplinary approach can foster a more comprehensive understanding of the mechanisms linking metabolic changes to neurological outcomes. Such collaborations could lead to novel therapeutic interventions that target specific metabolic pathways altered in post-TBI patients, offering innovative strategies for managing secondary injuries.
Another important direction involves the validation of identified biomarkers in diverse patient populations, considering various demographic and clinical factors such as age, sex, and injury severity. This will help determine the generalizability of metabolomic findings and their applicability across different contexts. Large-scale, multicenter studies could provide robust datasets for confirming the clinical utility of these biomarkers and establishing standardized protocols for their use in clinical settings.
The integration of metabolomic profiling with other omics technologies, such as proteomics and transcriptomics, may yield a multi-dimensional view of the biological processes involved in TBI. By building comprehensive biological networks that link metabolomic data to changes at the protein and gene levels, researchers can gain deeper insights into the pathophysiology of TBI and identify new therapeutic targets.
Additionally, the exploration of the gut-brain axis in the context of TBI is a burgeoning area of research. The composition of gut microbiota has been shown to influence neurological outcomes and may modulate metabolite profiles in CSF. Investigating the interplay between metabolic changes in CSF and gut microbiome could illuminate novel pathways and potential interventions for improving recovery from TBI.
As research in this field progresses, it is crucial to focus not only on the identification of biomarkers but also on their actionable insights. Education surrounding the implementation of metabolomic findings into clinical strategies will be essential for ensuring that the advancements made in research translate into improved patient care and outcomes. Engaging with healthcare professionals and stakeholders early in the research process will help align scientific discoveries with clinical realities, fostering an environment where metabolomics can substantially impact the management of TBI.
Overall, the future of metabolomics in understanding TBI is promising, with multifaceted research directions that may lead to significant advancements in personalized medicine, novel therapeutic strategies, and enhanced patient management in traumatic brain injury.