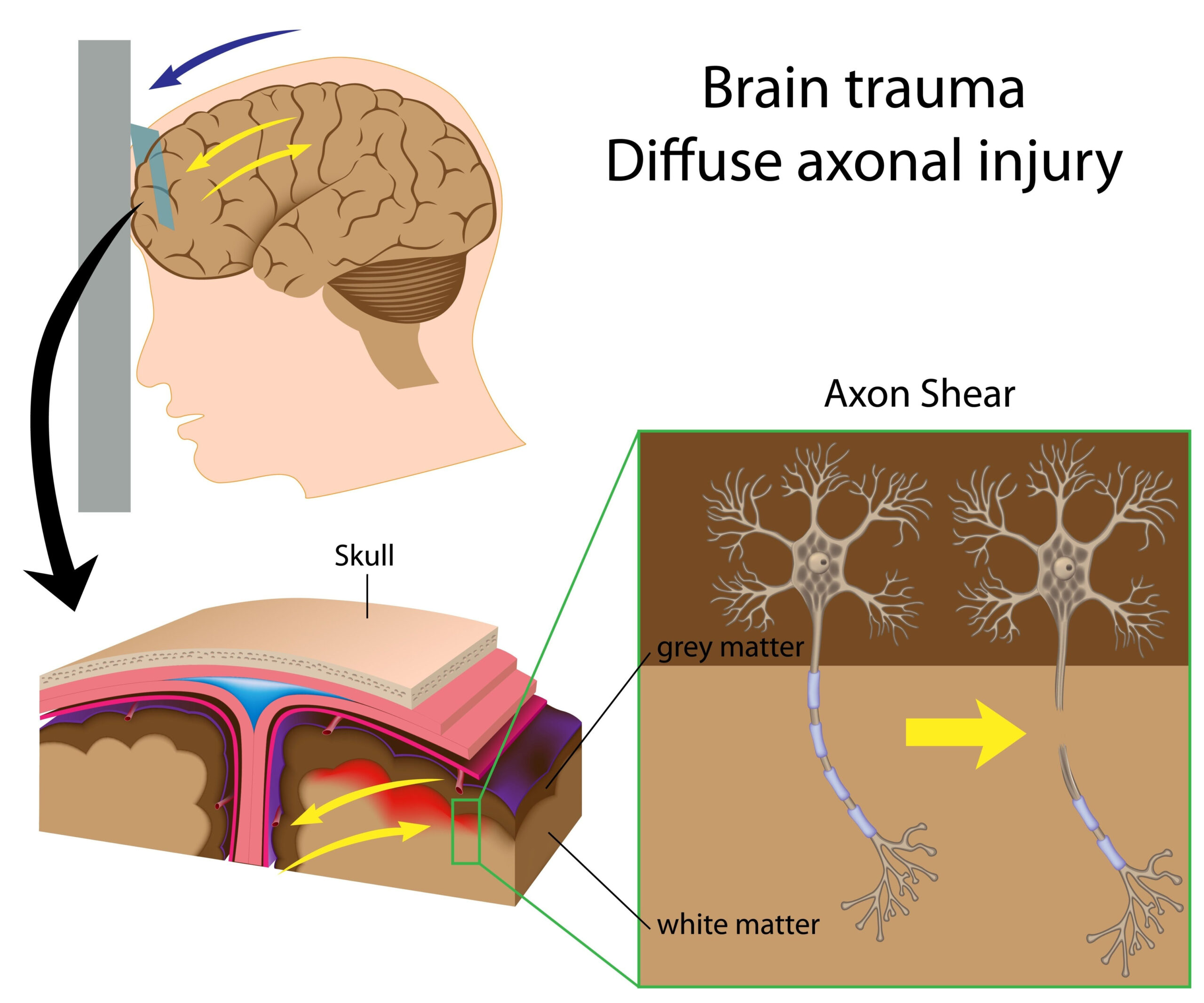
Study Overview
The research investigates the utilization of advanced brain organoid platforms to explore the impacts of traumatic brain injury (TBI) resulting from repeated blast exposures. Traumatic brain injuries are increasingly recognized as significant public health issues, particularly among military personnel and civilians exposed to blast waves from explosives. The study highlights the pressing need to better understand the mechanisms underlying TBI and the long-term consequences of such injuries, given the complexities involved in studying the human brain in vivo.
To address this challenge, researchers have developed brain organoids—miniature, simplified models of the brain created from human stem cells. These organoids can mimic the structural and functional characteristics of actual brain tissue, enabling scientists to observe how repetitive blast forces affect neural development and function in a controlled environment. By leveraging these innovative biological models, the study aims to deepen the understanding of TBI by assessing cellular responses and potential therapeutic interventions.
This work seeks to provide valuable insights into the pathophysiological changes that occur due to repetitive blast exposure, aiming to bridge the gap between bench-side research and clinical applications. Through an integrative approach combining both experimental and analytical techniques, the study aspires to foster the development of targeted therapies and improve strategies for the prevention and rehabilitation of TBI in affected populations.
Methodology
The research employed a detailed and multifaceted approach to explore the effects of repeated blast exposure on brain organoids derived from human pluripotent stem cells. The brain organoids were generated using a method known as embryoid body formation followed by guided neurogenesis, allowing them to develop into structures that closely resemble specific regions of the human brain, such as the cortical and hippocampal areas. This methodology provides a more authentic representation of human brain tissue compared to traditional animal models, enabling researchers to conduct more relevant studies of human neurological responses.
To simulate blast conditions, the researchers utilized a custom-designed shock tube to deliver controlled blast waves to the organoids. This experimental setup allowed for the precise application of varying intensities and durations of blast exposure, providing a robust framework for understanding how these factors impact neural integrity and function. The organoids were subjected to multiple blast events to replicate the cumulative effects observed in individuals experiencing repeated traumatic brain injuries. Following exposure, a range of techniques was employed to assess the resultant morphological and functional changes in the brain organoids.
Post-blast organoid analysis included imaging techniques, such as immunofluorescence, to visualize neuronal development and cell viability, as well as high-throughput transcriptomic profiling to uncover gene expression changes associated with blast exposure. These methods enabled the identification of specific molecular pathways and cellular responses activated by the traumatic insults. Additionally, behavioral assays were performed on organoid-derived neuronal networks to evaluate functional outcomes, assessing alterations in synaptic activity and network connectivity in response to the blasts.
Data were quantitatively analyzed using sophisticated computational tools to interpret the biological significance of the findings. By integrating these various methodologies, the study was able to produce a comprehensive picture of the responses elicited by blast exposures, revealing insights into both immediate effects and longer-term adaptations at the cellular and network levels within the organoid models. This rigorous experimental design forms the backbone of the research, allowing for robust comparisons and interpretations of results in the context of traumatic brain injury research.
Key Findings
The study yielded several significant findings that enhance our understanding of the effects of traumatic brain injury caused by repeated blast exposures, utilizing the advanced brain organoid models. One of the primary outcomes revealed that exposure to repeated blast waves led to notable morphological changes in the brain organoids. Following exposure, a marked decrease in neuronal density was observed, particularly in layers representing cortical structures. This suggests that repetitive trauma can compromise the development and maintenance of neurons, which are critical for cognitive functions.
In addition to structural alterations, the researchers documented functional impairments within the organoids. Electrophysiological assessments demonstrated reduced synaptic activity in neuronal networks derived from the organoids subjected to blast exposure. Specifically, there were significant disruptions in neuronal firing patterns and synaptic transmission, indicating that repeated blasts could impede communication between neurons, a vital process for brain function. These alterations in synaptic activity hint at the potential for long-term cognitive deficits that may arise from repeated traumatic exposures.
On a molecular level, high-throughput transcriptomic analysis uncovered significant changes in gene expression profiles post-exposure. In particular, the expression of genes associated with neuroinflammation and apoptosis was upregulated, suggesting that the response to blast exposure may involve inflammatory processes that could lead to neuronal cell death. The identification of these molecular pathways provides crucial insights into the biological mechanisms underlying the observed phenotypic changes, positioning the research to inform potential therapeutic targets for mitigating TBI effects.
Moreover, behavioral assays indicated that blast exposure affected the network-level activity of the organoid-derived neurons, leading to less robust network connectivity and poorer performance in tasks designed to assess neuronal network function. Such findings underscore the detrimental impact of cumulative blast exposure on the integrative capabilities of neuronal circuits, potentially mirroring the cognitive impairments seen in individuals suffering from chronic effects of TBI.
In summary, the research highlighted several critical findings: repeated blast exposure leads to significant morphological and functional impairments in brain organoids, mediated by inflammatory and apoptotic pathways. These results not only validate the utility of brain organoids in exploring TBIs but also set a foundation for future studies aimed at developing targeted therapies to address the neurobiological consequences of blast injuries. The implications of these findings extend beyond laboratory settings, emphasizing the need for effective strategies to protect and rehabilitate populations at risk for TBI.
Strengths and Limitations
The study presents several strengths that contribute to its importance in the field of traumatic brain injury research. One of the most notable advantages is the use of human brain organoids, which provide a more accurate and ethically sound model of human brain tissue compared to traditional animal models. This approach allows for a better representation of human neural development and pathology, promoting insights that are directly relevant to human health. The ability of these organoids to be generated from pluripotent stem cells means that they can be personalized to reflect the genetic backgrounds of different individuals, thereby enhancing the translational potential of the research.
Additionally, the sophisticated experimental methodology that includes controlled blasts simulates real-world scenarios faced by individuals exposed to explosive blasts. This level of control allows researchers to systematically vary the intensity and duration of exposure, enabling a robust examination of how these variables influence neuronal health and function. The combination of advanced imaging techniques and high-throughput transcriptomic profiling further enriches the data set, providing a multidimensional view of the biological responses to traumatic insults. Such a comprehensive approach ensures that both morphological and functional aspects of neuronal health are evaluated, yielding a rich tapestry of findings that deepen our understanding of TBI.
However, the study also has limitations that need to be acknowledged. One major constraint is the scale of the brain organoids, which, despite their advanced design, cannot fully replicate the complexities of the human brain in vivo. Critical interactions occurring within the larger brain architecture, such as those involving different brain regions or systemic responses, are not entirely captured within these miniaturized models. Furthermore, the short exposure timeframes used in laboratory settings may not adequately reflect the cumulative and prolonged nature of blast exposure experienced in real-world scenarios, potentially limiting the applicability of findings to long-term effects.
There is also the inherent challenge of generalizing results from organoid models to human patients. While organoids exhibit compelling similarities to human brain tissue, individual genetic and epigenetic variations among different patients could lead to variability in TBI responses that are not fully accounted for within a homogenous group of organoids. Moreover, the functional assays conducted may not capture the full spectrum of cognitive and behavioral repercussions that would arise from TBI in living organisms, as these complex interactions are influenced by many factors including lifestyle, emotional state, and environmental context.
Another aspect that warrants consideration is the translational gap that can exist between preclinical findings and clinical application. Identifying specific molecular targets or pathways through studies in organoids is an important step, but translating these insights into effective treatments or preventative strategies will require extensive validation in clinical settings. This transition from bench to bedside involves further layers of complexity, including drug dosing, delivery mechanisms, and potential side effects, which will need to be addressed in future studies.
In summary, while this research showcases several methodological strengths and provides vital insights into the effects of repeated blast exposure on brain function, acknowledging its limitations highlights the ongoing challenges and areas for future exploration in the realm of traumatic brain injury research. The findings pave the way for advancements in understanding the neurobiological mechanisms at play and creating effective therapeutic strategies to mitigate the impact of such injuries on affected populations.